how does a car battery work physics
The chemical reaction that powers a primary cell is one way. When you insert the key in your cars ignition and turn the switch or push the button to ON a signal is sent to the cars battery.

How To Test A Car Battery Youtube
P 600 A 72 V P 4320 W.

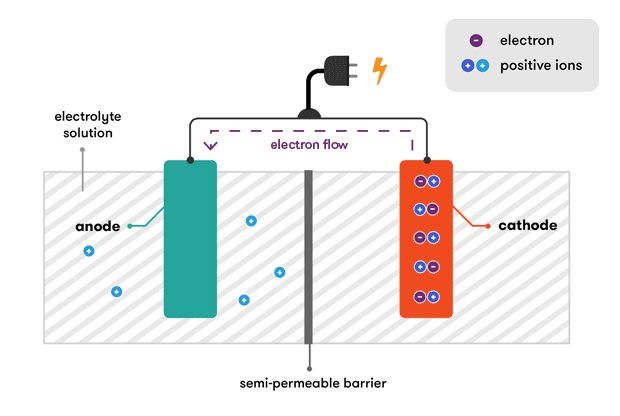
. A set of connected cells is called a battery. A reaction at the negative electrode whereby ions give up electrons at the electrode and. The separator blocks the flow of electrons inside the battery.
An electrical cell is a device used to produce electricity. The flow of electrons provides an electric current that can be used to do work. The chemical reactions in a battery involve the flow of electrons from one material electrode to another through an external circuit.
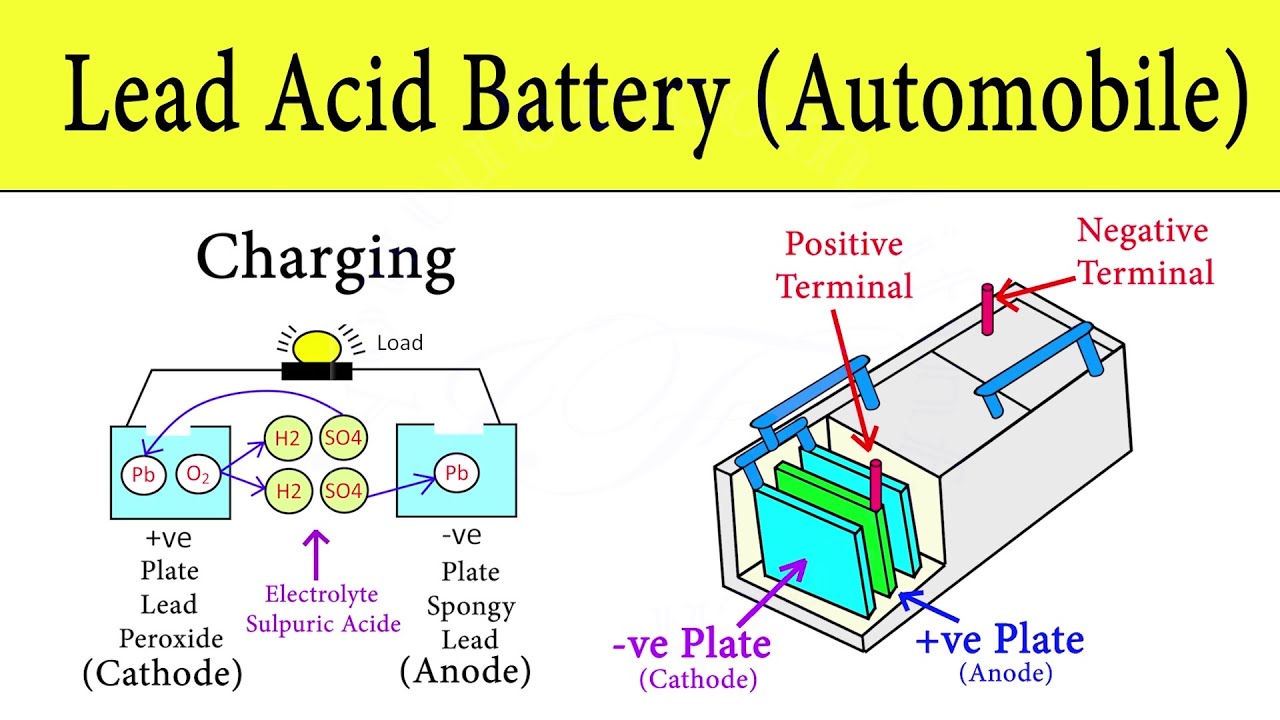
The lead dioxide plate creates ions and lead sulphate. I Current Expressed in amperes. A system such as a battery favors a state of lower enthalpy and it reaches that by two chemical reactions happening.
A car uses quite a lot of electricity to work the ignition and other electrical equipment. These batteries only work in one direction transforming chemical energy to electrical energy. A battery keeps the energy system of your vehicle sustainable.
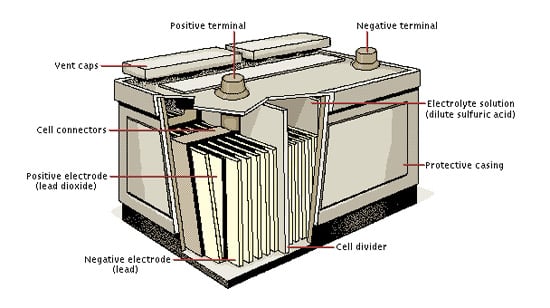
The paste is a lead oxide mixture that creates both lead dioxide and sponge lead. The battery has pairs of lead plates immersed in a mixture of sulphuric acid and distilled water. To balance the flow of electrons charged.
But in other types of batteries the reaction can be reversed. The plates are connected at the top by a cast-on strap that is welded to the plates. Stick two different metals into an electrolyte then connect them through an outer circuit and you get a tug-of-war going on between them.
A further reaction takes place creating electrons which then move quickly around the. Heres how the conventional lead-acid liquid cell car battery worksThe purpose of a car battery is to provide electricity to start the engine spark for ign. The general way that a battery works is that when an electronic circuit is connected to the battery electrons are allowed to flow.
A battery is a device that stores chemical energy and converts it to electrical energy. The movement of the lithium ions creates free electrons in the anode which creates a charge at the positive current collector. One or more cells are used as the batteries to produce.
The electrodes in the battery contain atoms of certain conducting materials. The battery is first used to start the engine and it does this by providing electricity to a small electrical motor known as the starter motor. To kickstart the chemical reactions in the battery you just connect a wire between its negative and positive terminals and a steady stream of electrons a current is produced as the reactions get under way.
The element consists of stacked alternating positive and negative plates. This electrical power is delivered to the starter to crank the engine. Once the motor is running an alternator supplies current to recharge the battery.
A battery starts up the ignition system of your vehicle. The starter motor engages a small gear onto the. The plates within each cell are arranged in alternating layers for a total of 16 components.
Inside the battery are 3 important things. All of the parts of the battery work together to make the flashlight light up. All of the positive plates in a cell are.
The electrical current then flows from the current collector through a device being powered cell phone computer etc to the negative current collector. Hydrogen H Oxygen O 2 Lead Pb Sulfur S Connection of an external consumer starts the chemical reaction in the battery. P Power expressed in watts.
V Electromotive force expressed in volts. This causes the voltages of each battery to add. Each cell contains a series of electrodes or plates.
These ions subsequently react with the lead plate making hydrogen and lead sulphate. A car battery is actually 6 smaller batteries that are lined up in series. To find the power of a car battery we multiply the CCA number by 72 volts.
The battery also provides power to the. The positive plates of the battery are constructed from lead peroxide PbO2. So batteries are just devices that convert chemical energy into electricity.
A reaction at the positive electrode whereby ions receive electrons from the electrode. One of the metals wins out and pulls electrons from the other through the outer circuitand that flow of electrons from one metal to the other is how a battery powers the circuit. The elements fit into the individual cells of each battery.
If an electrical appliance like a light bulb motor or. Half of the plates are connected to each terminal. Batteries come in two basic types.
When the reaction runs in its spontaneous. In this electro-chemical process four materials react with each other. A battery transfers initial currents of energy over to an alternate performance system of your vehicle an alternator.
The electrolyte a mixture of sulfuric acid H 2 SO 4 and. Once the chemicals are exhausted the battery is effectively dead. Rechargeable batteries like the kind in your cellphone or in your car are designed so that electrical energy from an outside source the charger that you plug into the wall or the dynamo.
T Time expressed in hours. Upon receiving this signal the car battery converts chemical energy into electrical energy. When we work or run the stored chemical energy in our body is converted into heat or kinetic energy.
A car battery stores energy in chemical form and converts it into electrical energy. The formula for determining battery energy is. There are 2 connectors that go out of the.
The negative plates of the battery are pure lead in a soft sponge-like state. Batteries are made up of one or more cells each containing a positive electrode negative electrode and an electrolyte. A battery stores chemical material for conversion to electrical energy.
If the power came from an ordinary battery it would soon run downSo a car has a rechargeable battery and a charging system to keep it topped up. I think that the voltage difference is. Sports cars and light trucks require higher cranking amps ranging from 700 to 1000 A.
E Pt VIt. A reaction starts when the plates become submerged in the sulphuric battery acid. It also provides electricity to the ignition system to start the combustion of fuel.
The typical car battery is found in the engine bay of the car. A few car battery companies include Diehard and Trojan. For instance in an alkaline battery.
In contrast the chemical reaction in a secondary cell is reversible. Most modern cars require relatively low cold cranking amps that range from 400 to 600.
Electric Vehicle Battery Pollution
How Does A Car Battery Work Mach 1 Services
The Lead Acid Car Battery Schoolworkhelper
Lead Acid Battery How Car Battery Works Automobile Battery Working Principle Animation Youtube

Battery Working Principle How Does A Battery Work Electrical4u

Rough Science Recharge A Battery Challenge Pbs

Maintenance Of Lead Acid Battery Electrical4u

How A Car Battery Works The Engineering Mindset

Anatomy Of An Auto Battery Battery Basics Car Battery Small House Design Automotive Mechanic

How A Car Battery Works Basic Working Principle Youtube

How A Car Battery Works The Engineering Mindset
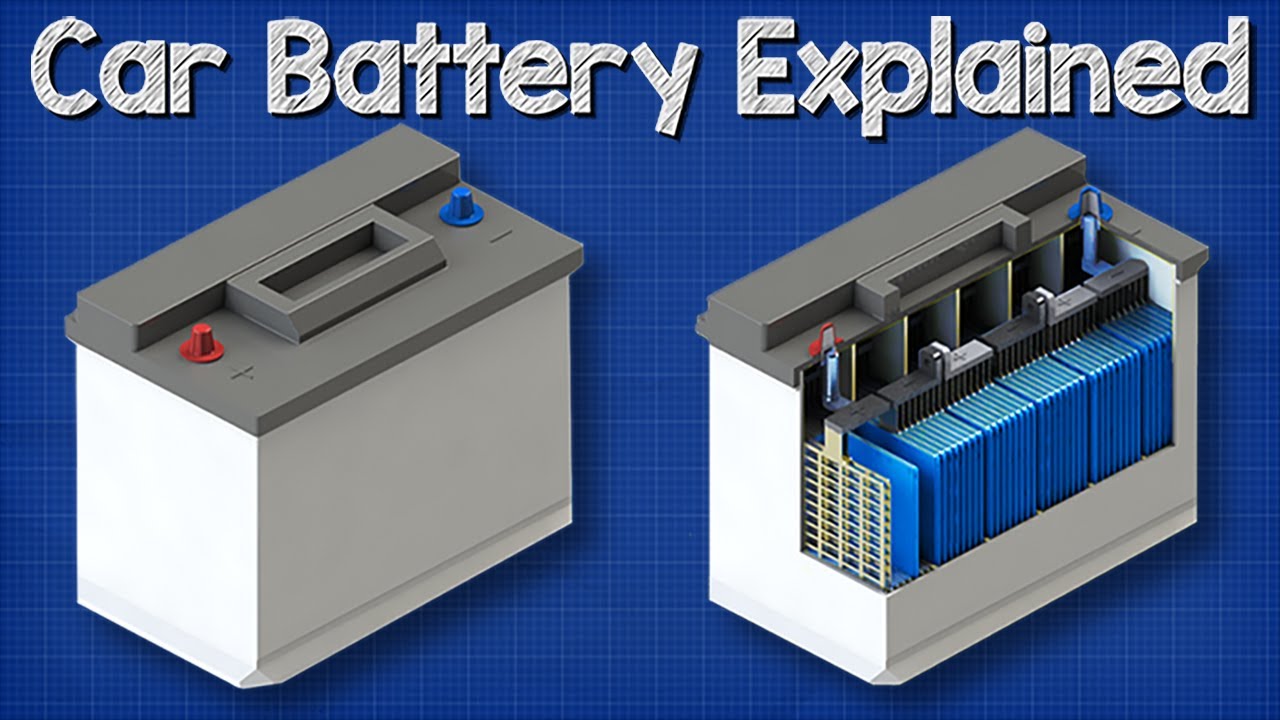
How A Car Battery Works Basic Working Principle Youtube
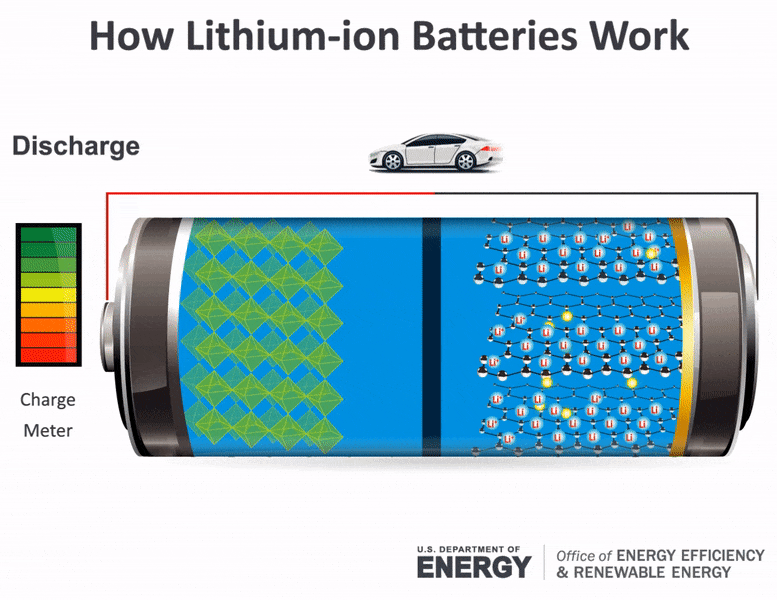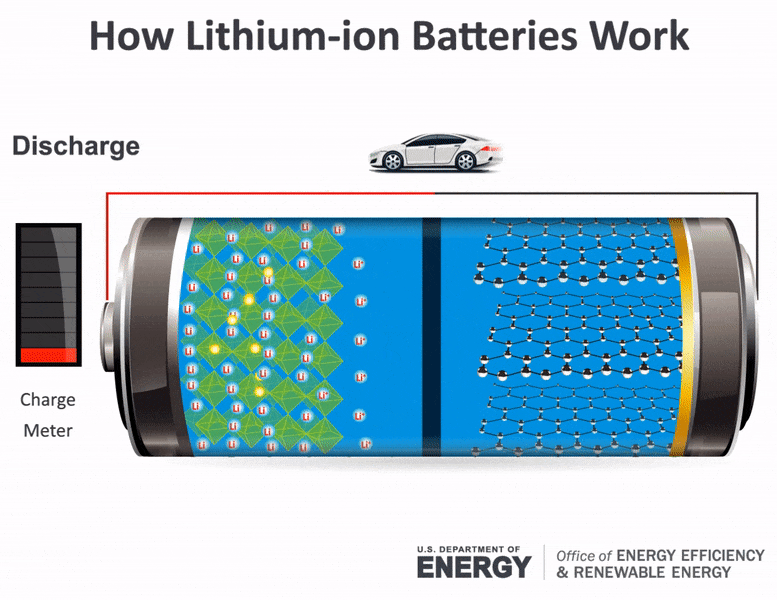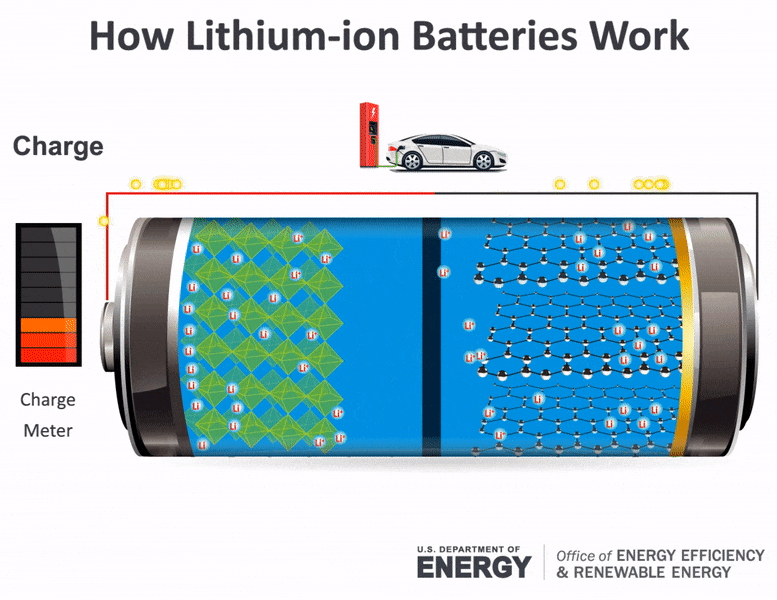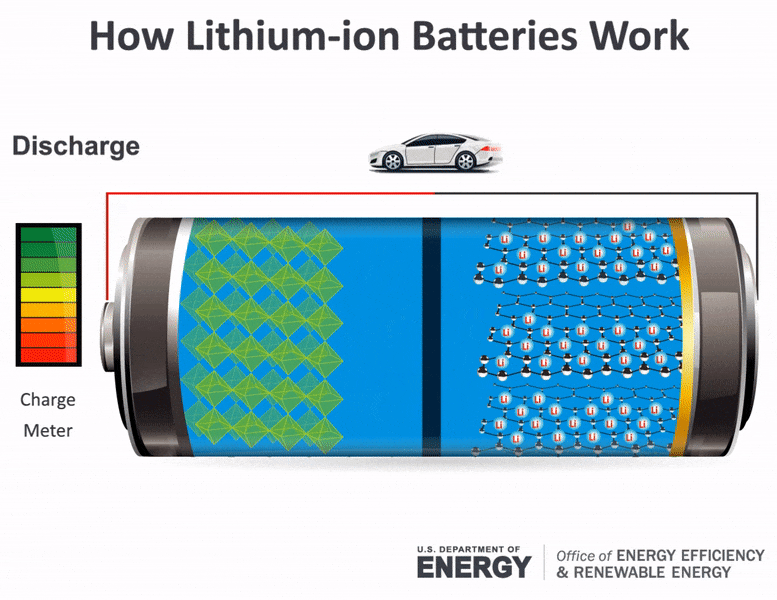
Science Made Simple What Are Batteries And How Do They Work

What Is Lead Acid Battery Working Construction Charging With Videos
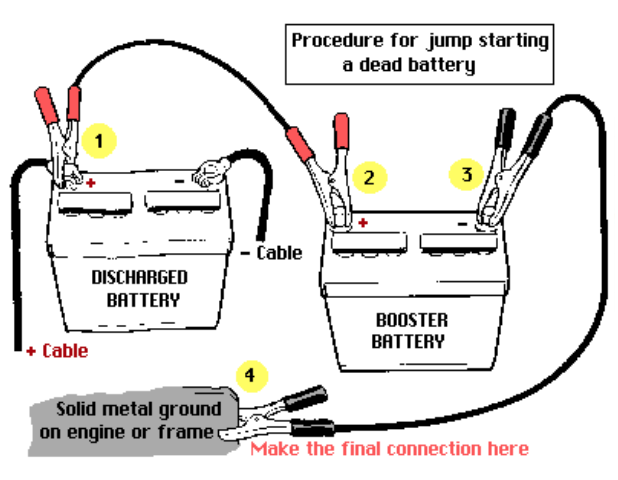
Solved Car Battery Application Of Kirchoff S Rules You Chegg Com

Working Of Lead Acid Battery Lead Acid Secondary Storage Battery Electrical4u